
South Africa charts a new frontier by rolling out a twice-yearly injection which is almost 100% effective in preventing HIV

Lower prices needed for new HIV prevention medicine in Brazil

Cambodia becomes the second country in the Asia Pacific to offer long-acting PrEP
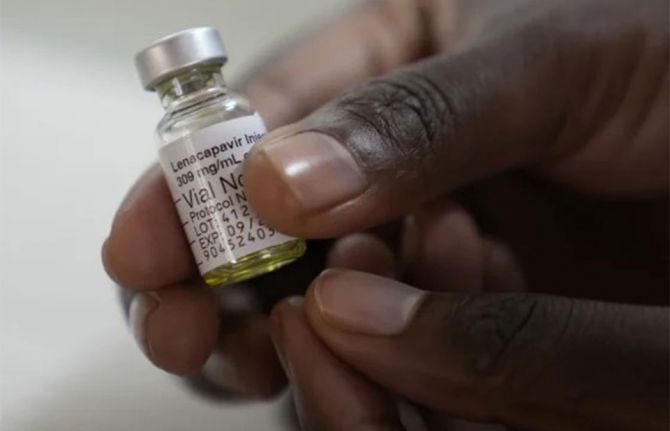
UNAIDS urges Gilead to drop price of new HIV prevention shot

UNAIDS calls on leaders at Davos to commit to rapid global access to revolutionary new long-acting HIV medicines

Global leaders in the HIV response call for access to long-acting medicines

PrEP for her: Cambodia, Indonesia, Papua New Guinea and the Philippines prepare to introduce the Dapivirine ring to help prevent HIV
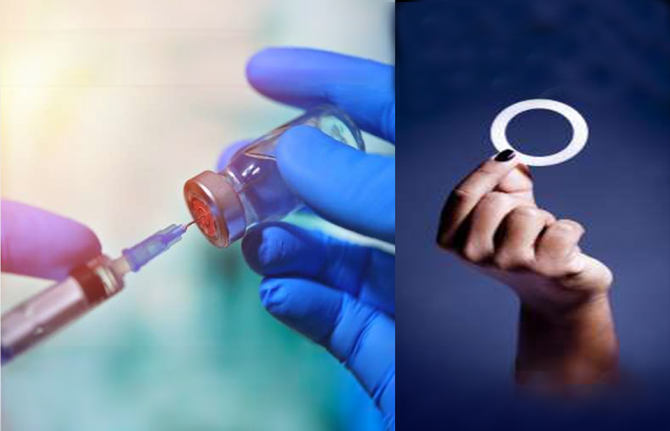
New long-acting HIV prevention options for women and girls in an era of choice

UNAIDS response to ViiV’s announcement on increasing production of long-acting cabotegravir

UNAIDS response to Gilead’s announcement on signing voluntary licensing agreements on lenacapavir with six generic manufacturers

New HIV drug can only offer hope of ending AIDS if all have access, UNAIDS says

Brazil hosts the announcement of the Global Council on Inequality, AIDS and Pandemics
UNAIDS welcomes the announcement by Medicines Patent Pool (MPP) and ViiV of three licenses signed with generic manufacturers for long-acting PrEP, and urges further urgent action by ViiV

Delays in global, affordable access to long-acting, injectable HIV medicines would cost lives, say AIDS campaigners
UNAIDS responds to EU approval of a long acting HIV treatment option: “To end AIDS, share technology.”
UNAIDS welcomes ViiV’s agreement to enable generic production of long acting PrEP to 90 countries
UNAIDS responds to Viiv’s announcement on the licensing of long-acting Cabotegravir

UNAIDS welcomes the approval of long-acting injectable cabotegravir as a pre-exposure prophylaxis for HIV prevention
