



Feature Story
PrEP for her: Cambodia, Indonesia, Papua New Guinea and the Philippines prepare to introduce the Dapivirine ring to help prevent HIV
22 November 2024
22 November 2024 22 November 2024The only HIV prevention that Elena Felix knew of was condoms. But condoms were not something that she was able to make use of, and she contracted HIV. Thirty years after her diagnosis, she’s helped conduct research to determine whether women in the Philippines would use a more confidential tool, and one that does not need a man to agree, to lower women’s risk of HIV infection.
“We hear from women that some partners insist on not using condoms. We hear cases too of rape. Women need protection that does not depend on men” the Association of Positive Women Advocates founder explained.
The Dapivirine Vaginal Ring or DVR was given the green light by the World Health Organization for women at high risk of contracting HIV in 2021. Unlike other types of pre-exposure prophylaxis (PrEP), this one is exclusively for women. It is a silicone vaginal ring that is inserted and worn for 28 days before being replaced. It releases an antiretroviral drug locally, reducing the risk of HIV infection through vaginal sex by half.
Since its introduction, the technology was made available in several (11) African countries. And with good reason. Around two-thirds of new HIV infections in Eastern and southern Africa and Western and central Africa are among adolescent girls and women. The combination prevention strategies implemented in these two regions have super-charged progress, driving the global 39% decline in new infections since 2010.
But the Asia Pacific picture is quite different.
“This region has an HIV prevention crisis,” Eamonn Murphy, UNAIDS Regional Director for Asia Pacific and Eastern Europe Central Asia said. “And I am not speaking only of the countries where new infections have doubled, tripled or increased six times since 2010. The average regional decline in new infections is far too slow. At 13% it has virtually flatlined.”
He was speaking to a group of community, government, research and development partners from Cambodia, Indonesia, Papua New Guinea and the Philippines who met from November 11 – 12 in Bangkok. Findings were disseminated from a DVR acceptability and feasibility study conducted by ThinkPlace, and a discussion held on next steps. UNAIDS and the World Health Organization (WHO) are providing technical support for this initiative. The Australia Department of Foreign Affairs and Trade (DFAT) funded the research as part of its ongoing support for prevention work in the region.
Seven percent of new infections in Asia Pacific are among sex workers while 12 percent occur among the intimate partners of key populations. Angeli Achrekar, UNAIDS Deputy Executive Director, called for women in Asia and the Pacific to be provided more HIV prevention options.
“Choice is the way to go!” Ms Achrekar stressed. “Providing options in prevention tools and service delivery increases overall use and results. We must ensure that people have access and that they are supported with the appropriate policies and enabling environment. The ring has great potential to be empowering as an additional choice for women, including in Asia Pacific.”
A person newly acquires HIV in the Asia Pacific region every two minutes. Despite this, the overall momentum on rolling out pre-exposure prophylaxis (PrEP) options has been sluggish. At the end of 2023 there were just 204,000 PrEP users in this region, 98% short of the 8,200,000 target by 2025. The vast majority of those on PrEP were men.
ThinkPlace Regional Director, Elliot Duffy, revealed that overall, the studies found women have high interest in this discreet, woman-controlled prevention method. Sex workers in the four countries sought the DVR given their high vulnerability to sexual violence. And in all countries the sex workers indicated that they would want to access the DVR through community-based health services or their local healthcare facilities. The research also found that healthcare providers in the four countries were enthusiastic about offering this new prevention option.
“The number one barrier is the extent to which women understand how the ring would fit. Many had questions like, “would it be lost in my body? Will I feel pain? Will I be able to have sex?’ Some women worried about a partner thinking they distrusted them,” Mr Duffy explained. “The DVR is not immune to the challenges of other HIV programs and continued effort is needed to increase awareness, generate demand and create services that are accessible.”
Already the research findings have resulted in the introduction and phased implementation of the DVR into 2024 – 2026 Global Fund grant implementation for Cambodia and Indonesia. Cambodia has begun pilot testing. At the meeting the four country teams developed plans to guide their next steps, including on further research, legal and policy reviews, regulatory approvals and community system strengthening.
DFAT Health Adviser, Joshua Metcalf-Wallach, emphasized that as stakeholders switched gears from research to rollout, they should keep communities in the driver’s seat.
“Our Indo-Pacific prevention work has shown that HIV services work best when they are key population- and community-led. As we expand prevention options for women, let us be guided by their needs and demands,” he ended.
Our work
Region/country

Feature Story
New long-acting HIV prevention options for women and girls in an era of choice
14 October 2024
14 October 2024 14 October 2024New long-acting technologies are changing the HIV prevention landscape. In recent years, innovation in pre-exposure prophylaxis (PrEP) has accelerated. Long acting injectable cabotegravir and the dapivirine vaginal ring as innovative formulations of PrEP have already joined oral PrEP containing tenofovir as WHO-recommended effective and acceptable options for HIV prevention, and there are additional antiretroviral-based options on the immediate horizon. These options complement other effective, non-antiretroviral-based HIV prevention products including condoms and lubricants, and harm reduction strategies.
This year clinical trial results for PURPOSE 1 and 2 showed the high prevention effectiveness of the 6-monthly long-acting injectable drug, lenacapavir for cisgender adolescent girls and women, cisgender men and transgender women. Among the cisgender adolescent girls and women participating in the trial, no HIV acquisitions were recorded during 12 months of follow-up among the women who received injectable lenacapavir. The Global HIV Prevention Coalition (GPC), UNAIDS and other partners called on Gilead Sciences to accelerate their efforts in ensuring that it is made available, accessible and cost effective especially to low- and middle-income countries. This twice-yearly injection is a promising option and offers increased choice, discretion and convenience for people who may benefit from HIV prevention.
In October 2024, at the Research for Prevention (R4P) conference in Peru, the Population Council announced phase 1 trial results from IPM 054, showing that the three-month dapivirine ring is as safe as the currently available one-month ring with similar levels of drug release. The 3-month ring like the 1-month ring is a woman-controlled option but would be more cost effective (an estimated 60% reduction in cost per user) and potentially an even more convenient HIV prevention option for women and adolescent girls.
“We need to follow the science, and the science has shown us that by making a range of effective HIV prevention options available and accessible, we can stop HIV transmission and drop new infections by addressing biomedical, behavioral and structural drivers simultaneously. Ending AIDS remains a political and financial choice”, says Angeli Achrekar, UNAIDS Deputy Executive Director Programmes.
This complements a partnership announcement by the Global Fund and the Children’s Investment Fund Foundation (CIFF) of an USD 2 million initiative for 2024-2025 to purchase an estimated 150 000 dapivirine vaginal rings for use as PrEP in the Global Fund grant implementing countries. This would increase accessibility to one of the most discreet HIV prevention options for women and adolescent girls.
The World Health Organization (WHO) PrEP Implementation Tool Provider Module for Oral and Long Acting PrEP, launched in July 2024, integrates clinical service delivery guidance for the three WHO recommended PrEP products (oral PrEP, the dapivirine vaginal ring and long-acting injectable cabotegravir) by a range of different providers in clinical or community settings and emphasizes the importance of access and choice.
These strategic advancements align to the HIV Prevention Choice Manifesto For Women and Girls in Africa that calls for prevention options to be made choices and urges that research and development of new HIV prevention options actual choices, thereby empowering women and girls to take control of their health and bodies. It also emphasizes the importance of ongoing research and development of innovative HIV prevention methods.
“Adolescent girls and women are gaining access to an increasing range of safe and effective options. Scale-up of HIV prevention will depend on supporting access to choice, strong country leadership and an enabling environment. An HIV free future for girls is possible, but only if the global community comes together with ambitious plans to make this range of PrEP options available with speed, scale and equity,” says Mitchell Warren, Executive Director, AVAC and GPC, Co-chair.
The GPC co-convened, by UNAIDS and UNFPA, will continue to work with its partners to accelerate HIV prevention to achieve the global target of less than 370 000 new HIV infections annually by 2025.
About the GPC
In 2017, a global coalition of United Nations Member States, donors, civil society organizations, and implementers was established to support global efforts to accelerate HIV prevention. Membership includes 38 of the highest HIV-burden countries, UNAIDS Cosponsors, donors, civil society, and private sector organizations. The overarching goal of the Global HIV Prevention Coalition is to strengthen and sustain a political commitment to primary prevention by setting a common agenda among key policymakers, funders, and program implementers.

Press Statement
UNAIDS response to ViiV’s announcement on increasing production of long-acting cabotegravir
08 October 2024 08 October 2024GENEVA, 8 October 2024—Responding to ViiV’s announcement on long-acting cabotegravir , UNAIDS Executive Director Winnie Byanyima said:
“New HIV prevention medication, in the form of a long-acting injection, could transform the lives of people who struggle to take daily pills. The option of an injection that only needs to be taken once every few months is vital for people who face stigma when seen with pills, and those who are driven underground by criminalizing laws.
The people most in need of this long-acting option include adolescent girls, LGBTQ+ people, sex workers, and people who use drugs.
The arrival of long-acting injections is truly a game-changer – it can help prevent millions of new HIV infections.
But this will only happen if everyone who would benefit has access. When medicines are lifesaving, delays are fatal.
To ensure affordable pricing and worldwide availability for everyone who needs these medicines, enabling access to generic versions in all low- and middle-income countries is essential. But ViiV continues to lock out many low- and middle-income countries from this possibility. Shockingly, the company has even launched a legal challenge against Colombia for trying to access a generic version of another lifesaving HIV medicine, dolutegravir.
ViiV's announcement on increasing production of long-acting cabotegravir is a welcome first step, but their next steps must follow fast. It is not enough for ViiV to increase the number of doses up for sale.
I urge ViiV to show leadership on access to medicines now by announcing an affordable not-for-profit price, dropping its harmful legal challenge, and enabling all low and middle-income countries to access generic versions of its medicines.
That is how they can help ensure this scientific breakthrough fulfils its potential and how they can help bring an end to the AIDS pandemic."
/ENDS
Note: ViiV’s announcement can be read on their site at https://viivhealthcare.com/hiv-news-and-media/news/press-releases/2024/october/triple-annual-supply-of-long-acting-hiv-prep-for-low-and-middle-income-countries/
UNAIDS
The Joint United Nations Programme on HIV/AIDS (UNAIDS) leads and inspires the world to achieve its shared vision of zero new HIV infections, zero discrimination and zero AIDS-related deaths. UNAIDS unites the efforts of 11 UN organizations—UNHCR, UNICEF, WFP, UNDP, UNFPA, UNODC, UN Women, ILO, UNESCO, WHO and the World Bank—and works closely with global and national partners towards ending the AIDS epidemic by 2030 as part of the Sustainable Development Goals. Learn more at unaids.org and connect with us on Facebook, Twitter, Instagram and YouTube.

Press Statement
UNAIDS response to Gilead’s announcement on signing voluntary licensing agreements on lenacapavir with six generic manufacturers
02 October 2024 02 October 2024GENEVA, 2 October 2024—Responding to today’s announcement by Gilead on lenacapavir, UNAIDS Executive Director Winnie Byanyima said:
“We welcome Gilead’s announcement of licensing the break-through HIV medicine lenacapavir for generic production. To stem the tide of new infections, and protect people most at risk from HIV, including young women and people from marginalised communities, long-acting HIV medicines are vital. Lenacapavir, which requires only two injections per year, could be game changing – if all who would benefit can access it.
We applaud Gilead for licensing the medicine without waiting for registration, which should be the norm. We are battling a pandemic and the speed at which generic versions come to market will dictate whether this medicine can really be transformative. At UNAIDS we commit to doing all we can to speed up this process.
Including an African producer in Egypt is also very welcome.
Much more work is still urgently needed to ensure that no one who needs lenacapavir is left behind and that Gilead’s commitment to rapid, affordable access is fulfilled.
The exclusion of many middle-income countries from the licenses is deeply worrying and undermines the potential of this scientific breakthrough.
HIV prevention products need to be deployed where new HIV infections are highest – and right now, forty-one percent of new infections are in upper-middle income countries. UNAIDS urges Gilead to secure further licenses for access in all low and middle-income nations.
We welcome Gilead’s statement of commitment to non-profit pricing, but we had been waiting eagerly for a specific price. We urge Gilead to disclose it, and to provide full transparency on their costs. Respected researchers have shown it is possible to produce and sell lenacapavir for $100 per patient per year, falling to as little as $40.
Manufacturing this medicine in African countries with the highest HIV rates is crucial for sustainability and Gilead should include manufacturers in countries like South Africa where there is strong production capacity. We at UNAIDS stand ready to assist.
UNAIDS urges Gilead to secure further licenses for access in all low and middle-income nations.
We urge Gilead also to do all it can to make lenacapavir viable for treatment in low- and middle-income countries, including working together with researchers to test new combinations. Over 30 million people worldwide taking HIV treatment every day deserve long-acting options. We recognize that Gilead has included treatment use in the license, where some companies have not, but we urge that they remove the current limitation in the license to “heavily treatment-experienced patients.” To support scientists and manufacturers worldwide, licenses should not be limited to specific uses.
Leaving no one behind is how to unlock lenacapavir’s full potential, fulfil Gilead’s promise, protect a generation from HIV and bring forward the end of the AIDS pandemic."
Note: Gilead’s announcement can be read on their site at https://www.gilead.com/news/news-details/2024/gilead-signs-royalty-free-voluntary-licensing-agreements-with-six-generic-manufacturers-to-increase-access-to-lenacapavir-for-hiv-prevention-in-high-incidence-resource-limited-countries
UNAIDS
The Joint United Nations Programme on HIV/AIDS (UNAIDS) leads and inspires the world to achieve its shared vision of zero new HIV infections, zero discrimination and zero AIDS-related deaths. UNAIDS unites the efforts of 11 UN organizations—UNHCR, UNICEF, WFP, UNDP, UNFPA, UNODC, UN Women, ILO, UNESCO, WHO and the World Bank—and works closely with global and national partners towards ending the AIDS epidemic by 2030 as part of the Sustainable Development Goals. Learn more at unaids.org and connect with us on Facebook, Twitter, Instagram and YouTube.
Documents
2024 global AIDS report — The Urgency of Now: AIDS at a Crossroads
22 July 2024
This UNAIDS 2024 report brings together new data and case studies which demonstrate that the decisions and policy choices taken by world leaders this year will decide the fate of millions of lives and whether the world’s deadliest pandemic is overcome. Related links: Press release | Special web site | Executive summary | Fact sheet | Video playlist | Epidemiology slides | Data on HIV | Annex 2: Methods Regional profiles: Asia and the Pacific | Caribbean | Eastern Europe and Central Asia | Eastern and Southern Africa| Latin America | Middle East and North Africa | Western and Central Africa | Western and Central Europe and North America Thematic briefing notes: People living with HIV | Gay men and other men who have sex with men | Transgender people | Sex workers | People who inject drugs | People in prisons and other closed settings | Adolescent girls and young women | Other translations: German


Press Release
New HIV drug can only offer hope of ending AIDS if all have access, UNAIDS says
10 July 2024 10 July 2024GENEVA, 10 July 2024— UNAIDS has welcomed the release of Gilead Sciences’ trial results on the injectable long-acting HIV medicine Lenacapavir for HIV prevention. The result “provides hope of accelerating efforts to end AIDS”, UNAIDS says, “but only if Gilead ensures that all people who need it can have access to this game-changing medicine.”
The recent trial of the medicine among cis-gender women in Uganda and South Africa was so successful that it was halted early. Twice-yearly injections of Lenacapavir showed overwhelming efficacy for preventing HIV infections compared to standard oral preventative HIV medicines, known as pre-exposure prophylaxis (PrEP). Additional trials are ongoing in Argentina, Brazil, Mexico, Peru, South Africa, Thailand and the United States.
UNAIDS has welcomed the “exciting development,” and urged the company to allow generic production of Lenacapavir to all low- and middle-income countries by negotiating voluntary licensing agreements through the Medicines Patent Pool (MPP). The MPP is a UN-backed programme with extensive experience negotiating generics agreements between originators and generic pharmaceutical companies.
Gilead has not yet announced its plans for low and middle-income countries. However, UNAIDS is concerned that Gilead’s latest statement regarding its access strategy for low and middle-income countries mentions only “high incidence countries and resource limited countries” and makes no specific mention of upper-middle-income countries or the Medicines Patent Pool. Upper middle-income countries account for 41% of new HIV infections and 37% of all people living with HIV. These countries are home to millions who cannot afford the prices Gilead charges high-income countries.
“The success of Gilead’s recent Lenacapavir trial is an exciting development. While we still await regulatory approvals, normative guidance and results from the other ongoing trials, this news offers hope that we can enable everyone who would benefit, including especially the most marginalised communities, to have access to the help they need. Enabling equitable global access to new technologies can help get the world on track to end AIDS as a public health threat by 2030,” said Winnie Byanyima, Executive Director of UNAIDS. "However, it is concerning that Gilead’s latest announcement seems to mention neither upper-middle income countries, where people cannot afford anything like Lenacapavir’s current $42,250 price tag, nor a commitment to work with the UN-backed Medicines Patent Pool. Without these safeguards, it cannot be assured that this game-changing medicine will reach all those who need it."
Notes
Data in this press release comes from UNAIDS 2023 Epidemiological estimates (aidsinfo.unaids.org)
The UNAIDS Executive Director joined more than 300 experts and activists calling for a generic version of Lenacapavir to be licensed to all low and middle-income countries through the MPP, in a letter coordinated by the People’s Medicines Alliance: https://peoplesmedicines.org/wp-content/uploads/2024/05/Gilead-Open-Letter_May-2024.pdf
The AIDS Vaccine Advocacy Coalition provides an overview of the Lenacapavir for PrEP trials: https://avac.org/resource/infographic/an-overview-of-lenacapavir-for-prep-trials/
UNAIDS
The Joint United Nations Programme on HIV/AIDS (UNAIDS) leads and inspires the world to achieve its shared vision of zero new HIV infections, zero discrimination and zero AIDS-related deaths. UNAIDS unites the efforts of 11 UN organizations—UNHCR, UNICEF, WFP, UNDP, UNFPA, UNODC, UN Women, ILO, UNESCO, WHO and the World Bank—and works closely with global and national partners towards ending the AIDS epidemic by 2030 as part of the Sustainable Development Goals. Learn more at unaids.org and connect with us on Facebook, Twitter, Instagram and YouTube.



Press Release
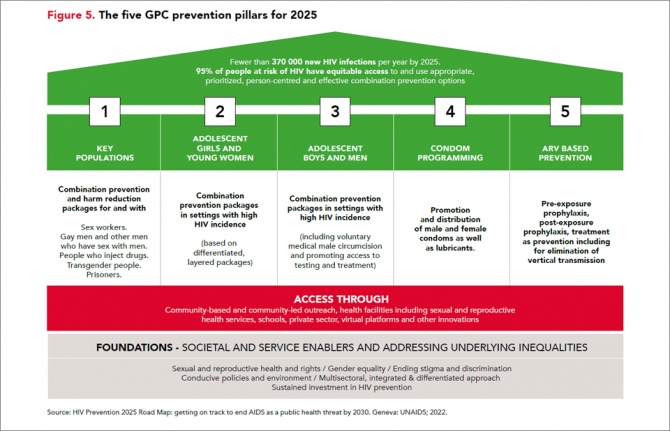
Reductions in new HIV infections in several Global HIV Prevention Coalition countries, but global progress needs to be accelerated
13 March 2024 13 March 202413 March 2024—A new report, HIV Prevention: From Crisis to Opportunity shows that HIV infections continue to decline in countries that are part of the Global HIV Prevention Coalition (GPC) faster than in the rest of the world.
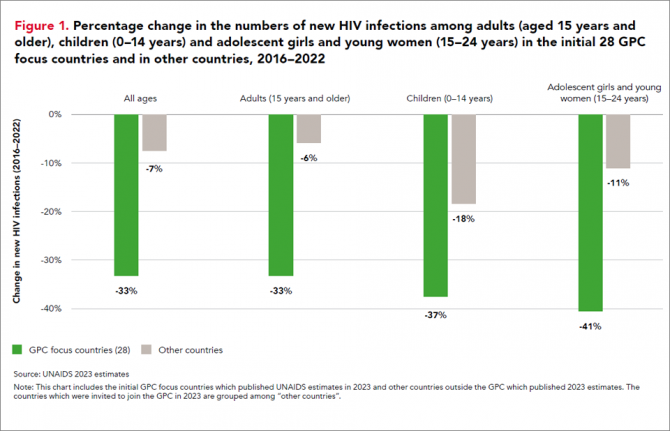
Eleven GPC focus countries have reduced their annual number of new HIV infections by at least 66% since 2010. By comparison, the average reduction in new HIV infections since 2010 globally is 38%. The GPC is a coalition of 38 countries working together to accelerate declines in new HIV infections to achieve the target of having 95% of the people who are at risk of HIV accessing effective combination prevention options.
The GPC countries that have prioritised primary prevention and treatment and that have focused on reaching people most at risk have secured the strongest consistent declines in new HIV infections.
Globally, progress in HIV prevention has been highly uneven and a majority of the world’s countries are not currently on track to achieve the 2025 targets. Indeed, several countries are experiencing prevention crises with low access to services and face record rising new HIV infections.
“The findings of this report offer crucial lessons for action,” said Angeli Achrekar, Deputy Executive Director Programme, UNAIDS. “The report shows that sustained political leadership, investment in effective HIV prevention programmes, and an enabling policy environment are crucial to end AIDS as a public health threat by 2030.”
Declines in new HIV infections have been boosted by the cumulative impact of combination HIV prevention options and increased access to antiretroviral treatment which has also increased viral suppression in people living with HIV. People who are on treatment and are virally supressed cannot transmit HIV.
“It’s remarkable to see what has been achieved in the AIDS response in the past 20 years. But the progress to date has not been equitable and is not yet sustainable, and we must never confuse progress with being sure of success,” said Mitchell Warren, GPC co-chair and Executive Director, AVAC. “Our progress is fragile, and what we’ve achieved today could slip away even more quickly than it was achieved if we let complacency take hold.”
Key populations and adolescent girls and young women are still at high risk of new infections
HIV incidence remains unacceptably high among populations where gaps in HIV prevention investments persist. This includes key populations in all regions globally and adolescent girls and young women in parts of sub-Saharan Africa.
Around 3100 young women and girls aged 15-24 became newly infected with HIV every week in sub-Saharan Africa in 2022 and HIV incidence declined less rapidly than it has among young men. Only 43% of the sub-national areas in which there is elevated HIV incidence among young women are being reached with dedicated prevention programmes for young women.
Although GPC countries have shown solid gains in reducing new HIV infections, challenges remain worldwide in reaching key populations most at risk of new HIV infections including men who have sex with men, sex workers and people who inject drugs. Every week, more than 11 000 new HIV infections occur among key populations and their sexual partners globally.
Only 44% of sex workers, 28% of gay men and other men who have sex with men, and 37% of people who inject drugs accessed two or more HIV prevention services in the previous three months according to median values reported by GPC countries ––against a target of 90%.
HIV prevention is being obstructed by shortfalls in prevention financing, and by punitive laws. Social stigma, violence, discrimination and social exclusion are barriers to key populations’ access to health-care services and information, exacerbating their risk of acquiring HIV. Law reform is a crucial enabler of prevention programmes. Protecting the human rights of everyone is vital for protecting the health of everyone.
Investments in both condom and voluntary medical male circumcision programmes, which are both effective in preventing HIV, have fallen in some of the countries with the largest HIV epidemics. In addition, breakthrough HIV prevention options such as pre-exposure prophylaxis (PrEP), medicine to prevent HIV, are still only available to a small fraction of the people who need them.
There are unprecedented opportunities for HIV prevention in 2024. There is a growing array of prevention options including existing tools and new long-acting prevention technologies, as well as country examples of how to implement prevention at scale and increase choices available to communities.
HIV Prevention programmes need to be at scale, efficient and equitable. The actions that are needed for success and sustainability are known, have been shown to work, and have been agreed: collaborate, follow science, tackle inequalities, protect everyone’s rights, let communities lead, and invest in what is needed. Sliding back on resourcing or inclusion would hurt everyone. Solidarity will benefit everyone. Communities, countries and international partners can prevent new infections – together.
UNAIDS
The Joint United Nations Programme on HIV/AIDS (UNAIDS) leads and inspires the world to achieve its shared vision of zero new HIV infections, zero discrimination and zero AIDS-related deaths. UNAIDS unites the efforts of 11 UN organizations—UNHCR, UNICEF, WFP, UNDP, UNFPA, UNODC, UN Women, ILO, UNESCO, WHO and the World Bank—and works closely with global and national partners towards ending the AIDS epidemic by 2030 as part of the Sustainable Development Goals. Learn more at unaids.org and connect with us on Facebook, Twitter, Instagram and YouTube.
Our work
Documents
HIV prevention: from crisis to opportunity — Key findings from the 2023 Global HIV Prevention Coalition scorecards
13 March 2024
The Global HIV Prevention Coalition focus countries are progressing unevenly towards the goal of reducing HIV infections to levels that would no longer constitute a public health threat. The biggest declines are occurring in eastern and southern Africa and, to a lesser degree, in western and central Africa. Expansion of access to effective ART, combined with an ongoing focus on primary prevention, are driving those achievements.
Documents
Summary — Let Communities Lead — UNAIDS World AIDS Day report 2023
28 November 2023
This report is not only a celebration of the critical role of communities. It is a call to action to decision-makers to fully support the life-saving work of communities and to clear away the barriers that stand in their way. Press release | Full report | Fact sheet | World AIDS Day 2023








